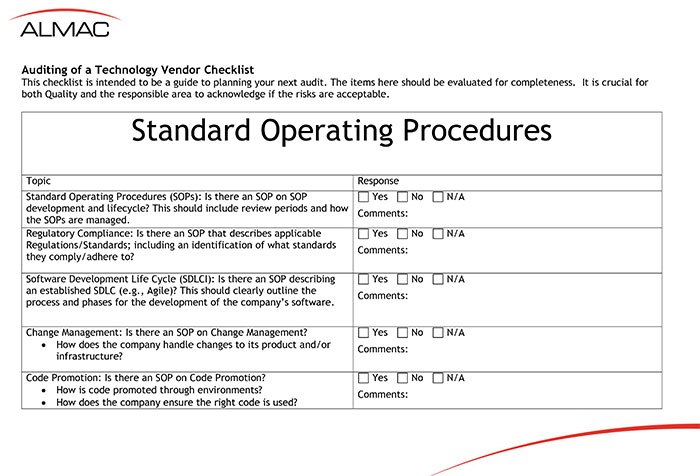
Auditing of a Technology Vendor Checklist
This comprehensive checklist will help you plan your next audit of a technology vendor. It also summarizes the points made during the Selecting and Auditing a Technology Vendor for Biopharmaceutical Development webinar (also available in the Almac Clinical University Library) and provides an excellent starting point to help you identify key areas and potential risks to your company and trial.
Download Now
Free Instant Access
*By submitting your information you acknowledge that you have read the privacy statement and you consent to our processing the data in accordance with that privacy statement. We may, from time to time, send you material relevant to your interests. If you change your mind at any time about wishing to receive material from us, you can send an email to [email protected]. Every email we send you will also include an unsubscribe link so you can unsubscribe from our marketing list.

